Introduction
Levine et al. [3] reported an increase of 30% in cancer detection rates by taking 2 consecutive sets of sextant biopsies at a single visit, with no reported increase in morbidity. However, others have shown that taking more biopsies without altering the angle of the biopsy needle or changing the regions of the prostate sampled may not improve cancer detection rates. Ravery et al. [4] took 10 systematic biopsies in 162 patients with suspected prostate cancer (PSA > 4 ng/mL, with or without an abnormal DRE). In addition to the standard sextant biopsy regimen, two further parasaggital biopsies were obtained bilaterally, one between the standard apical and mid-gland biopsy sites, the other between the mid-gland and basal biopsy sites. Five biopsies were taken from each side of the prostate, all in the same plane and at the same angle. The additional biopsies yielded only a 3% diagnostic improvement when compared with systematic sextant biopsies alone [4].
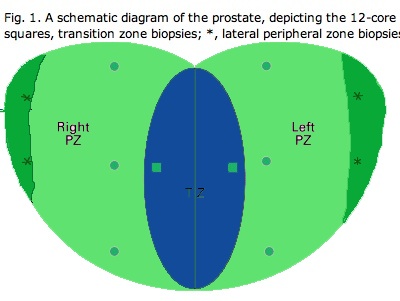
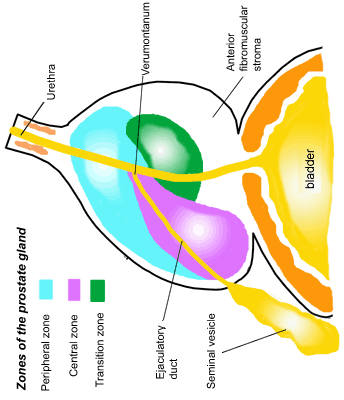
Most prostate cancers arise in the peripheral zone (PZ) [5], a region that may be compressed by the expanding transition zone (TZ) when there is significant BPH.
To improve PZ sampling it has been proposed that needle placement for systematic biopsies be directed more laterally [6], so that the biopsy tract traverses more of the PZ and encompasses the lateral PZ.
- In prospective studies, the addition of lateral PZ biopsies to the standard sextant protocol detected an additional 14–31% of cancers that would have remained undetected by the sextant method alone [7,8].
- The combination of sextant and lateral PZ biopsies significantly increased cancer detection while almost eliminating the need for lesion-directed biopsies [8].
Although most prostate cancers originate in the PZ, up to 24% may arise in the transition zone (TZ) [5] and would therefore be missed by any biopsy protocol that sampled only the PZ.
Isolated TZ tumours are thought to have a favourable prognosis, being of low Gleason grade, infrequently associated with prostatic intraepithelial neoplasia (PIN) [9] and often discovered incidentally at transurethral resection of the prostate (TURP).
Many of the published studies examining the issues surrounding TRUS-guided prostate biopsy have been conducted on asymptomatic men with screening-detected tumours and relatively low serum PSA (< 20 ng/mL) levels. In the UK, at present there is no national screening programme for prostate cancer (although a clinical trial has recently begun) and most tumours are detected on a 'case-finding' basis. In contemporary urological practice in the UK the largest group of patients referred with suspected prostate cancer are men presenting to their GP, or to a nurse-led prostate-assessment clinic, with bothersome LUTS and who subsequently undergo PSA testing. To determine the optimal biopsy strategy for such patients with suspected prostate cancer referred to our department for assessment, we undertook a prospective study to evaluate whether an extended-core biopsy protocol incorporating routine TZ biopsies and lateral PZ biopsies, in addition to the standard sextant technique, would improve the overall detection rate for prostate cancer.
Patients and methods
- The mean (sd, range) age of patients undergoing biopsy was 68.7 (8.2, 44–89) years. All patients consented to undergo an extended-core biopsy protocol before inserting the probe.
- The indications for TRUS were an elevated PSA (> 4.0 ng/mL) and/or an abnormal DRE.
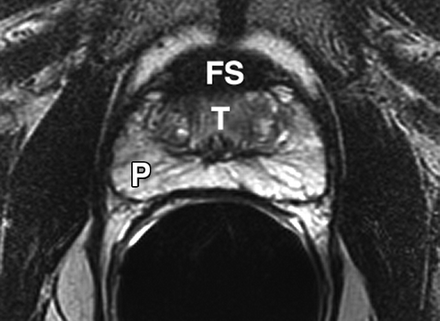
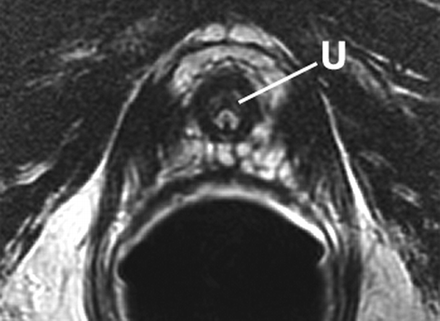
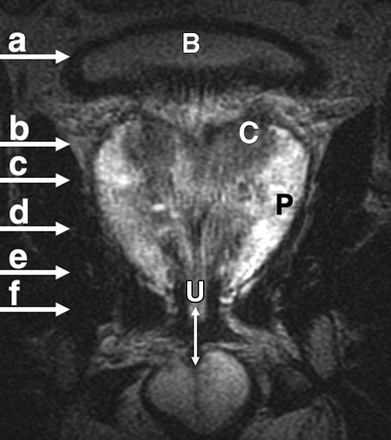
Sextant biopsies were taken routinely ≈ 1 cm apart in the parasaggital plane bilaterally, at the base, mid-gland and apical regions of the prostate, as described by Hodge et al. [1]. In addition, six further biopsies were obtained, two from the TZ and four from the lateral PZ, as depicted in Fig. 1. The TZ biopsies were taken at the level of the mid-gland where the TZ was most prominent. The lateral PZ biopsies were taken by positioning the probe just medial to the lateral edge of the prostate at the base and mid-gland regions bilaterally, as described by Chang et al. [8]. This method generally allowed any area of DRE abnormality or suspicious hypoechoic lesion noted on TRUS to be incorporated into the biopsy protocol. All patients underwent the same biopsy strategy with no variance for gland size. Biopsies were obtained using an 18 G core-biopsy needle mounted on a spring-loaded automatic biopsy gun. All patients were placed in the left lateral decubitus ('knee-chest') position and all were examined with no prior bowel preparation. The procedure was generally well tolerated and no patient required intravenous sedation or narcotic analgesia. All TRUS was undertaken by the same operators (D.R.G. and N.S.), either personally, or when supervising a higher urological trainee.
Biopsy cores taken from the sextant regions, TZ and lateral PZ were labelled and submitted separately in formalin-filled containers to the Department of Pathology, Sunderland Royal Hospital, where they were processed, paraffin-embedded and examined histologically for the presence of cancer by consultant pathologists. All patients were given a metronidazole (1 g) suppository after biopsy and were prescribed a 3-day course of amoxicillin/clavulinic acid, or ciprofloxacin if penicillin-allergic. PSA was measured before biopsy in all cases using the Immulite 2000® assay (Diagnostic Products Corp., Gwynedd, Wales, UK). When the final histological diagnoses were available, the outcome and cancer detection rates for the various anatomical regions biopsied were determined and compared. The histological diagnoses in the case of patients with biopsies negative for cancer were also recorded. Serum PSA levels and prostate volumes for men with positive and negative biopsies were compared using nonparametric analyses, with P < 0.5 considered significant. Data for minor complications of biopsy were not available, because they were self-limiting and treated by either the patient or their GP. However, complication rates for any patient with major morbidity after biopsy that required in-patient treatment were recorded accurately.
Results
493 patients
|
|
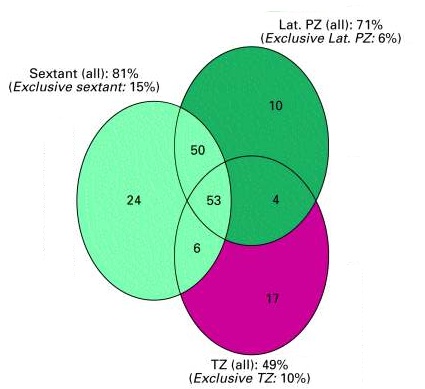 Fig. 4: Number of positive biopsies from each anatomical region (total number of positive biopsies = 164). Areas do not represent numbers of positive biopsy results. 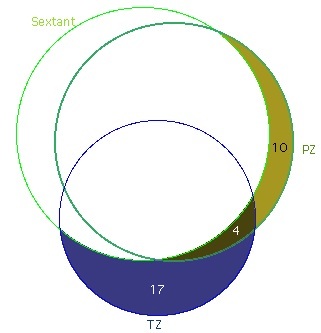 Fig. 4a: Cancers missed by routine sextant biopsies (total number of positive biopsies = 164). Areas represent numbers of positive biopsy results. |
|
To assess the effect of prostate volume on biopsy outcome,
- the 424 patients in whom volume-based measurements were available were stratified into those with total prostate volumes either above or below 50 mL.
- Volume-based measurements were available for 137 of the 164 men with positive biopsies and 74% had prostates of < 50 mL.
- Most (66%) of the additional tumours detected, that would have otherwise remained undetected with sextant biopsies, were in men with smaller (< 50 mL) prostates.
- A summary of the influence of gland volume on cancer detection rates for each biopsy strategy is given in Table 3.
- Although patients with tumours confined exclusively to the TZ had slightly higher serum PSA levels than patients with tumours detected elsewhere within the prostate, this difference was not significant (17.5 vs 13.9 ng/mL, P = 0.34).
- Also, there was no significant difference in total prostate or TZ volume between these groups.
- However, patients with isolated TZ tumours were significantly more likely to have serum PSA levels of > 10 ng/mL than patients with tumours in other regions of the prostate (Table 4).
Discussion
- Over the course of the study, 18 patients (3.6%) developed complications after biopsy serious enough to warrant hospital admission (Table 2).
- Most patients were discharged within 3 days although both patients with Gram-negative septicaemia required extended stays of 6 and 8 days, respectively.
- 8 patients required catheterization, 4 each (0.8%) for acute urinary retention and haematuria that required a bladder washout and overnight irrigation.
- All voided spontaneously after catheter removal.
Detection Rates
A greater awareness of prostate cancer by patients has lead to more men requesting a PSA test. Some are asymptomatic, but many undergo PSA testing as part of an evaluation for lower urinary tract symptoms (LUTS). This, in combination with recent government cancer directives, is likely to result in a significant increase in the numbers of men requiring prostate biopsy to exclude malignancy. The optimal biopsy protocol for such patients has not yet been determined, but it is unlikely that a 'one-biopsy protocol fits all' scenario will emerge. From the clinicians' and the patients' perspective, a biopsy protocol that maximises cancer detection with minimal discomfort and an acceptable complication rate is what is required.
- Some would argue that adopting an aggressive approach to prostate cancer detection by taking many biopsies risks detecting clinically insignificant disease.
- While that may be the case for a small proportion of tumours detected, most stage T1c prostate cancers have been shown to be clinically significant in terms of histological grade and tumour volume [16].
- From studies of patients undergoing repeat biopsy for suspected prostate cancer where an initial set of sextant biopsies was negative, routine sextant biopsies give false-negative results in 19–31% of cases [17–19].
- Taking more biopsy cores at the initial TRUS should improve cancer detection, reducing false-negative outcomes, patient anxiety, inconvenience and overall costs.
- Some have advocated reserving extended-core biopsies for men with larger prostates or for those undergoing repeat biopsy. However, mathematically, for a given volume of prostate cancer, the likelihood of detecting cancer increases as more biopsies are taken [20].
- Most additional tumours (66%) detected in the present series were in men with prostates of <50 mL (Table 3), justifying the use of the extended biopsy protocol even in patients with smaller prostates.
- Overall, the number of tumours detected [in extended-core biopsies] increased by 19% when the extended-core biopsy protocol was compared with the outcome of sextant biopsies alone. For the 493 men who underwent biopsy, this equates to a 6% increase in the rate of cancer detection from 27% (sextant only, 133/493) to 33% (extended biopsies, 164/493).
- This finding is in general agreement with other published extended biopsy protocols [7,21,22]. Although the present sampling technique may have differed from those in other reports, it is clear that prostate cancer detection can be significantly improved by taking more biopsies (within reason) and sampling regions of the prostate not incorporated in the routine sextant protocol.
Initially we were surprised at the relatively few additional tumours detected by lateral PZ biopsies, as many groups have reported a significant improvement in cancer detection rates with this technique [7,8,21]. However, the present detection rate of an additional 6% of tumours confined exclusively to the lateral PZ compares favourably with Ravery et al. [23], who noted a 6.6% overall improvement in prostate cancer detection with the addition of 4–6 lateral PZ biopsies, depending on prostate volume. In contrast, Naughton et al. [24] found no significant improvement (27% vs 26%) in cancer detection when a 12-core extended biopsy protocol incorporating the lateral PZ was prospectively compared with routine sextant biopsies alone.
TZ biopsies tend to cause greater discomfort than PZ biopsies as the needle needs to be advanced significantly further into the prostate. Accordingly, these biopsies were taken last during the extended-biopsy protocol, to ensure that patients tolerated the entire procedure.
- Given the low yield of routine TZ biopsies [12–15], it has been suggested that TZ biopsy be reserved for patients with previous negative biopsies undergoing repeat biopsy, where cancer detection rates can be increased by 10% [25].
- However, 21 (13%) additional cancers not diagnosed with sextant biopsies were detected with TZ biopsies at the time of the initial TRUS, 17 of which (10% of all cancers detected) were confined exclusively to the transition zone.
- It is unclear why the present detection rates for TZ cancer were higher than those reported elsewhere. In the absence of screening, we continue to see a full spectrum of presentation with prostate cancer, from clinically localized disease to locally advanced and metastatic prostate cancer. The incidence of isolated TZ cancer seems to be lower in screen-detected (asymptomatic) men than in patients presenting with LUTS and a raised PSA, who represent a significant proportion of men undergoing prostate biopsy in our unit.
- While we do not advocate that routine TZ biopsies be taken in all men with suspected prostate cancer, they may be appropriate for selected groups, such as those with serum PSA levels of > 10 ng/mL, as noted (Table 4).
- Evidence to support this approach comes from a study by Lui et al. [11], where a third of cancers detected in a subgoup of patients with a normal DRE but elevated PSA levels were confined exclusively to the TZ.
The incidence of major morbidity in the present series was 3.6%; this is somewhat higher than that reported in the only prospective study to examine the incidence of complications after TRUS-guided biopsy (in 128 men), where a major complication was defined as one requiring hospital admission [26]. All other complications were considered minor,
- the commonest being persistent haematuria (47%), which bore no relationship to the number of biopsies taken.
- Infectious complications (fever/rigors) were noted in 2.5% of patients but were treated on an outpatient basis.
- Only one patient required admission in that study for a seizure after a vasovagal episode.
- Similarly, major complications were classified in the present study as those requiring admission and inpatient treatment. No patient experienced a significant vasovagal episode but 2% of patients with infectious complications required admission for parenteral antibiotics.
- We have not prospectively compared complication rates for 6 vs 12 biopsies but others have reported no significant increase in pain or serious morbidity when men undergoing 12 biopsies were compared with men undergoing routine sextant biopsies [27].
- In another study of 119 men undergoing a 5-region extended-core biopsy protocol, no serious complications were reported, although 80% of patients reported self-limiting haematuria [7].
- Extensive sampling of the prostate may result in acute urinary retention caused by transient prostatic oedema precipitating bladder outlet obstruction (BOO).
- The incidence of acute urinary retention in the present series (0.8%) is similar to that reported by Rodriguez and Terris [26] (mean number of biopsies 8.26, range 6–13) but significantly less than the 10% incidence noted by Borborglu et al. [28] (mean number of biopsies 22.5, range 15–31) after an extensive repeat-biopsy protocol.
- In the present series there was no relationship between biopsy outcome (positive or negative) and the incidence of major complications.
The present extended-core prostate biopsy protocol incorporating sextant, lateral PZ and TZ regions was better than sextant biopsies in terms of cancer detection; it was well tolerated by patients and associated with a relatively low incidence of major complications. In selected men with suspected prostate cancer, routine TZ biopsy should be considered, particularly where the serum PSA level is > 10 ng/mL.