Hydroxychloroquine and Lyme
Int Microbiol 2002 Mar;5(1):25-31
Brorson O, Brorson SH.
Department of Microbiology, Vestfold Sentralsykehus, Tonsberg, Norway.
In this work the susceptibility of mobile and cystic forms of Borrelia
burgdorferi to hydroxychloroquine (HCQ) was studied.
-
The minimal bactericidal concentration (MBC) of HCQ against the mobile
spirochetes was
-
> 32 microg/ml at 37 degrees C, and
-
> 128 microg/ml at 30 degrees C.
-
Incubation with HCQ significantly reduced the conversion of mobile spirochetes
to cystic forms.
- When incubated at 37 degrees C, the MBC was
-
> 8 microg/ml for young biologically active cysts (1-day old), but
it was
-
> 32 microg/ml for old cysts (1-week old).
(compare with effective concentrations
and toxic concentration given in GG)
-
Acridine orange staining, dark-field microscopy and transmission electron
microscopy revealed that the contents of the cysts were partly degraded
when the concentration of HCQ was > or = MBC.
-
At high concentrations of HCQ (256 microg/ml) about 95% of the cysts were
ruptured (250 microg/ml is the toxic concentration).
-
When the concentration of HCQ was > or = MBC, core structures did not develop
inside the cysts, and the amount of RNA in these cysts decreased significantly.
-
Spirochetal structures inside the cysts dissolved in the presence of high
concentrations of HCQ.
-
When the concentration of HCQ was > or = MBC, the core structures inside
the cysts were eliminated.
These observations may be valuable in the treatment of resistant infections
caused by B. burgdorferi, and suggest that a combination of HCQ and a macrolide
antibiotic could eradicate both cystic and mobile forms of B. burgdorferi.
PMID: 12102233 [PubMed - in process]
Hydroxychloroquine - Chloroquine
Pharmacokinetic Data
Source: Goodman & Gilman's "The Pharmacological Basis of Therapeutics", 9th edition (1996)
CHLOROQUINEa
AVAILABILITY (ORAL) (%): 89 ± 16
URINARY EXCRETION (%): 61 ± 4
BOUND IN PLASMA (%): 61 ± 9; ´ rheumatoid arthritis
CLEARANCE (CL/F, ml * min-1 * kg-1): 1.8 ± 0.4b
VOL. DIST. (Vss/F, liters/kg): 115 ± 61b
HALF-LIFE (t1/2, hours): 41 ± 14 daysb,c
EFFECTIVE CONCENTRATIONS: 15
ng/mld; 30 ng/mle
TOXIC CONCENTRATIONS: 0.25 mg/mlf
Abbreviations:
a Active metabolite, desethylchloroquine, accounts for 20 ± 3%
of urinary excretion; t1/2 = 15 ± 6 days.
b Blood CL/F, Vss/F, and t1/2; blood-to-plasma concentration
ratio = 9.
c Shorter half-lives reported previously when sampling stopped after
1 month.
d Plasmodium vivax.
e Plasmodium falciparum.
f Diplopia; dizziness.
Reference:
White, N.J. Clinical pharmacokinetics of antimalarial drugs. Clin.
Pharmacokinet., 1985, 10:187-215.
(added by J. Gruber)
-
F = body weight,
-
Vss = steady state volume of distribution
-
t1/2 = elimination half life
-
CL/F units = ml of blood compartment / (min kg of body weight)
-
Enter body weight (e.g. 70 kg) and you have CL = "total body clearance"
in units ml of blood compartment / min.
Half Life
Chloroquine is safer when given orally because the rates of absorption
and distribution are more closely matched;
-
peak plasma levels are achieved in about 3 to 5 hours after dosing by this
route.
The half-life of chloroquine increases
as plasma levels decline, reflecting the transition from slow distribution
to even slower elimination from extensive tissue stores.
-
The terminal half-life ranges from 30 to 60 days, and
-
traces of the drug can be found in the urine for years after a therapeutic
regimen.
Volume of Distribution V
V = amount of drug in body / C
The
-
plasma volume of a typical 70-kg man is 3 liters,
-
blood volume is about 5.5 liters,
-
extracellular fluid volume outside the plasma is 12 liters, and
-
the volume of total body water is approximately 42 liters.
However, many drugs exhibit volumes of distribution far in excess of these
values(, indicative of the fact that these drugs have preferentially entered
compartments other than 1. - 4.).
Example:
If 500 mg of digoxin were in the body of a 70-kg subject, a plasma
concentration C of approximately 0.7 ng/ml would be observed. Dividing
the amount of drug in the body (500 mg) by the plasma concentration (0.7
ng/ml) yields a volume of distribution for digoxin of about 700 liters,
or a value ten times greater than the total body volume of a 70-kg man.
In fact, digoxin, which is relatively hydrophobic, distributes preferentially
to muscle and adipose tissue and to its specific receptors, leaving a very
small amount of drug in the plasma.
Enrichment in Tissue, Brain,
Spinal Cord
(from Product
Information for chloroquine phosphate (ARALENE) distributed by Sanofi
Winthrop Pharmaceuticals, Park Ave, New York, NY 10016, USA)
In animals the concentration 200 ... 700 times the plasma concentration
may be found in
-
liver
-
kidney
-
spleen
-
lung.
Chloroquine is also enriched in leukocytes.
The concentration in
is only 10 ...30 times the concentration in plasma.
Explanations of usage of terms Clearance, Volume of Distribution,
Half-Life
(added by J. Gruber)
Following GG, General Principles, Chapter "Clinical Pharmacokinetics"
in Leslie Benet "Pharmacokinetics" .
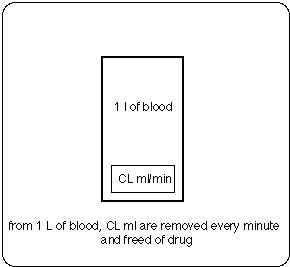
CL = Clearance from blood compartment (ml per minute)
(the notion used is that every minute a volume CL (ml) is removed
(and freed of drug) from the volume of distribution Vss)
Example.
In GG-Appendix II, the "plasma clearance" for cephalexin
is reported as CL/F = 4.3 ml min-1 kg-1,
with 91% of the drug excreted unchanged in the
urine.
For a 70-kg man, the "total body clearance from plasma" CL
= 300 ml/min,
with renal clearance accounting for 91% of this
elimination.
In other words, the kidney is able to excrete cephalexin
at a rate such that approximately 273 ml of plasma would be freed of drug
per minute.
Because clearance usually is assumed to remain constant
in a stable patient, the total rate of elimination of cephalexin will depend
on the concentration of drug in the plasma (equation
1-2).
useful definitions:
-
Clearance
CL = (rate of elimination by specified route) / (Concentration in specified
compartment)
-
Elimination half life
t1/2 = ln2/k = ln2 Vss/CL
Thus
CL = k Vss and
k = CL/Vss (= fraction of Vss removed per minute)
-
Diff. eq. describing elimination:
dm/dt = - k m
(in GG k m is called "rate of elimination")
(equation 1-2)
rate of elimination = C CL =
= C k Vss =
= k C Vss = k m
with the following nomenclature:
C = concentration of drug in blood (g drug/ml blood)
CL = clearance ((ml blood / l volume of distribution) / minute)
k = elimination constant,
m = mass of drug (g)
rate of elimination = dm/dt = change of mass of drug in Vss per unit
time
Dosage
(1) Dosage for malaria
(from Product Information for ARALEN Phosphate (chloroquine
phosphate), distributed by Sanofi
Winthrop Pharmaceuticals, Park Ave, New York, NY 10016, USA)
Dosage may be taken in as multiples of D = 5 mg base per kg body weight,
but D should not exceed 300 mg base:
-
first dose: 2 D,
-
6 hours after the first dose: D.
-
18 hours after the second dose: D,
-
24 hours after the third dose: D .
So, the total dose is: 5 D = 25 mg base per
kg body weight.
Explanation:
-
200 mg of hydroxychloroquine sulfate (Plaquenil) is equivalent to 155 mg
base.
-
500 mg of chloroquine phosphate (Aralen) is equivalent to 300 mg base
(2) Estimate of dosage to achieve a 32 microg/ml
concentration of free Chloroquine in blood (enrichment 1, i.e. no enrichment), tissue
(enrichment 200), brain (enrichment 10)
During approx. 2 half-lives the intake has to fill up the volume of distribution
Vss = 100 l/kg 70 kg = 7 103 l.
If
-
100 % of the drug was bioavailable and
-
100 % of it free (not bound to other molecules),
the total amount of the drug taken in has to be intake1
intake1 = 32 microg/ml Vss = 224 g of base
Because
-
availability is roughly 100 % and
-
roughly half is bound to plasma proteins,
the intake has to be 2 intake1:
necessary intake = 2 intake1 = 448 g.
The dosage estimate is 448 g/ (2 t1/2).
Although the high volume of distribution tells us that chloroquine distributes
into compartments other than 1. - 4, and the concentration
in those compartments is probably higher than
the concentrations in blood, the GG data do not tell us how low the concentration in blood can be to yield a concentration of
32 microg/ml in Bb cyst infected tissue.
Using the enrichment data from Sanofi
and assuming an elimination half life t1/2 = 41 + 14 days = 55 days, we will
arrive at the following approximate dosages (numbers in parentheses ( ) apply to a half life t1/2 = 41 - 14 days = 27 days)
-
dosage = 4000 (8000) mg/day for 32 microgram/ml in blood,
-
dosage = 20 (40) mg/day for 32 microgram/ml in tissue,
-
dosage = 400 (800) mg/day for 32 microgram/ml in brain.
The dosage to obtain 32 microgram/ml in blood is higher than the dose (approximately 1000 mg/day) recommended for adults having malaria (see also Medline literature search, keywords "hydroxychloroquine/adverse effects"[All Fields] AND dosage).
When less than 4000 (8000) mg per day are taken in, the blood compartment might not be bactericidal for mobile spirochetes, if the Brorsons' in vitro data are applicable to the in vivo situation. One has to keep in mind: This is a very rough dose estimate that
aimes at providing us a hint as to whether or not HCQ can be discussed as a drug against Lyme disease.
Further Reading
Version: August 19, 2008
Joachim Gruber
URL of this page is http://www.lymenet.de/literatur/hydroxychloroquine.htm
Home of this server is: http://www.lymenet.de
